Clinical Studies
“In an effort to streamline and modernize clinical investigations”
the FDA, in September 2013, issued final guidance which promotes capturing
source data in electronic form, Traditionally, site personnel have not entered
clinical data into EDC as source at point-of-care (POC) due to the lack of
responsiveness of EDC systems that operate by exchanging data and metadata
to their host servers via the web.
- NESS has spent the last 8 years studying the process by which clinical study data is collected, verified, cleaned, analyzed and submitted for approval.
- We have found that the most efficient way to collect and clean clinical study data is by utilizing ‘native’ mobile technology at POC.
- Only NESS has been able to create a development system, NEForm™, to quickly build, test and deploy clinical studies collecting both electronic Case Report Form (eCRF) and Clinical Outcomes Assessment (COA) data including electronic Patient Reported Outcomes (ePRO) and Clinical Reported Outcomes (ClinRO) data at the investigator site and ePRO and Observer Reported Outcomes (ObsRO) data at home (away from the site) utilizing all ‘native’ applications that support bring-your-own-device (BYOD) technologies.
- Current BYOD technologies supported are Apple IOS®, Android® and Windows® smartphones, tablets, phablets and Windows® PCs and laptops.
Pharmaceutical
- The Pharmaceutical industry faces increased pressure to bring new and more effective life enhancing chemical entities to market at a faster pace while clinical development costs continue to soar.
- Changing regulatory and payer environments are requiring companies to provide real-world evidence (RWE) and patient outcomes data in support of product claims.
- More than 50% of physicians are now deploying real-time, mobile technology in their clinical practice at point-of-care.
- Clinical investigators are beginning to demand that the same real-time, mobile tools used for recording data in their clinical practice are also used for the collection of data when their site participates in a clinical study.
- NESS NEForm reduces costs, increases efficiency and makes it practical to collect clinical data from medical centers, individual physician practices and directly from patients and subjects in clinical studies anywhere in the world, at any time, on any device.
Back to top
Early-Phase Clinical Trials
- Early-Phase Ia, Ib and IIa trials require go-live deployment in 4 weeks or less.
- Total costs need to be significantly lower than the costs for mid-phase and late-phase trials.
- Shorter length of trials require quick mid-study change turnaround times.
- NEForm Study Builder allows NESS personnel to build, verify and deploys studies in weeks not months.
- Most mid-study changes are built and deployed in hours not days
- NESS pricing model takes into account the short time span and limited budgets of early-phase trials
Back to top
Mid-Phase Clinical Trials
- NEForm saves time, saves costs and increase efficiency for phase II and III clinical trials.
- NEForm utilizes eSource data capture, virtually eliminating the need for site Source Data Verification (SDV) and Risked-Base Monitoring (RBM).
- A clinical monitors’ valuable time may be spent evaluating safety and compliance, rather than verifying if the blood pressure recorded in the database was really 125/80.
- NEForm captures source data accurately and contemporaneously. The patient is at the site when important edit checks fire for determining patient eligibility or alerting investigators and coordinators of any safety issues, so that appropriate decisions may be made and actions may be taken.
- NEForm is both site and patient-centric and supports both ePRO data collection at the site and away from the site and site eCRF data collection.
- Clinical trials involving both eCRF and ePRO date collection are more efficient and cost-effective with the NEForm integrated system.
Vaccine Trials
- The exposure and incidence of infectious diseases is increasing worldwide; driving the growth rate in the market for new vaccines to be nearly double the growth rate for that of new drugs.
- Access to the internet, in many parts of the world is nonexistent or slow therefore, the majority of vaccine trials are still dependent on paper data collection.
- Collecting vaccine data on paper is far from contemporaneous and involves much effort to ensure accuracy and security.
- NEForm collects vaccine data offline on mobile devices without the need for an internet connection
- With NEForm data can be collected in a remote village in Africa, India, China and South America and in any language Hindi, Cantonese, Swahili, Arara do Para etc.
CNS Trials
- Rater Training, eCRF and ePRO data capture on the same NEForm platform
- Utilizes an Apple IPad, Android or Windows Tablet
- Data is integrated and resides in the same database.
- Cost effective and efficient
Veterinary Trials
- Collect animal health data offline on Apple IPad, Android or Windows Tablets without internet connection.
- Collect animal health data anywhere, in a barn, in a kennel, out on a pasture, fast and unobtrusively.
- Collect animal health caregiver assessment data.
Back to top
Late-Phase, Post-Marketing, Health Economic & Outcomes (HEOR) and Registry Studies
- NEForm captures accurate, contemporaneous, branded, language-specific, ePRO and eCRF data, online or offline on any device, at anytime and anywhere with or without an internet connection.
- Utilizing NEForm for both eCRF and ePRO data collection eliminates the time, delay, cost and chore associated with collecting paper CRFs and paper patient questionnaires, transporting them and scanning them or double entering them for database storage and data cleaning.
- NEForm eliminates the cost and redundancy of collecting disconnected eCRF and ePRO data on multiple vendor electronic systems.
- By eliminating site SDV, an eSource system saves 25% - 30% of the cost of a clinical studies.
- The use of BYOD for patient ePRO data collection dramatically decreases the time and the cost for commissioning, testing, deploying and maintaining application specific data collection devices.
- The ability to scale is of particular importance for large registration studies.
- With web-based data collection systems, as studies grow, page turn rates degrade and users become increasingly frustrated. Over time, the shear amount of data and the sharing of bandwidth by additional users result in a decrease in system responsiveness.
- NEForm collects data offline and therefore distributes the work load across all study participants devices. All edit checks including cross-form and cross-visit edit checks are fired offline and local to the device and do not require sending data up to the server in order to check logic.
- With NEForm there is a dramatic decrease in up and down internet traffic. The response time for the first 10 users enrolled in a large global study or registry is the same as when the study expands to over 40,000 users.
Back to top
Biotechnology
- Many biotechnology companies are small with limited investment capital and therefore rely on the most efficient and cost-effective data collection, cleaning and reporting technologies to maximize their return for their clinical dollars.
- Biotechnology company studies are designed to net the cleanest results, with the smallest patient population, in the shortest amount of time.
- Project oversight and reliance on knowledgeable partners who understand their need to move quickly and dynamically while staying within study budgets are of prime importance.
- NEForm’s ability to collect eCRF and ePRO data on the same platform, to perform complex stratified randomization, to track and manage drug/ device supply and delivery, to provide milestone-driven, site payments, to import data from laboratory and other external sources, to export data to Safety, CTMS and other systems, and to extract data in many different formats maximizes the return on biotechnology companies resource dollars.
Back to top
Medical Devices
- Medical device companies are interested in maximizing the return on their clinical study dollars to get the cleanest data utilizing the smallest patient enrollment.
- Although, most medical device studies tend to be less complex than drug studies, long follow periods require a flexible cost model for hosting of the clinical data and supporting the system users.
- NESS has experience working with medical device clients and we are fully aware of the need to be efficient at data collection, and budget-conscious of hosting and support services.
Back to top
Academic Medical & Government Funded Studies
- NESS delivers complex study designs for academic medical and government studies, which are often financed by fixed public, private and commercial grants.
- In many cases, the end product of an Investigator Initiated Trial (ITT) is a peer reviewed abstract or scientific publication whose data help advance medical and scientific knowledge.
- NEForm makes data exports available in over 20 different statistics analysis formats including SAS, Excel, SPSS, Sigma, Stata etc.
Back to top
CROs
- NESS understands the need to deliver, on time and on budget to our CRO partners, the most efficient and cost-effective data collection solution for their clients’ studies.
- NESS has spent years studying how clinical investigator site users interact with mobile systems. Based on what we have learned, we built NEForm to optimize the site user experience.
- If investigator sites are happy, sponsors are happy and if sponsors are happy, CROs are happy. Our CRO partners know they can rely on NESS to understand their business and their clients business and to always over deliver.
Back to top
Medical
In order to affect more positive patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has begun to base a percentage of reimbursement that hospitals, physicians, nursing homes, kidney dialysis centers and other medical providers receive on Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys. These CAHPS surveys ask consumers and patients to report on and evaluate their experiences with healthcare providers.
Hospital Rounding
- Hospital (H)-CAHPS surveys, which are patient satisfaction surveys occurring post-discharge, give no opportunity for providers to address service failures with contemporaneous corrective action.
- Prospective surveys taken with NEForm HIPAA compliant, bedside Rounding System are a more effective way to allow the surfacing and response to problems prior to patient discharge resulting in:
- better patient outcomes
- a reduction in call button usage
- a reduced burden on nursing staff
- an average increase in post-discharge H-CAHPS scores
- In hospital and other clinical environments the internet is not always available and in certain locations its use is restricted.
- Unlike purely web-based technology, NEForm Rounding supports 'native' applications that collect data offline and empower users to enter data in real-time at speeds equal to or faster than that of putting pen-to-paper.
Back to top
Dental
NEForm has been used to improve mobile dental care for years in both research and clinical work. Mobile dentistry is a growing industry as professionals bring dental care
to people who need it. Regulations vary from state to state and web connectivity is unreliable from site to site. So dentists and hygenists need a solution
that is highly adaptable and works independent of an internet connection.
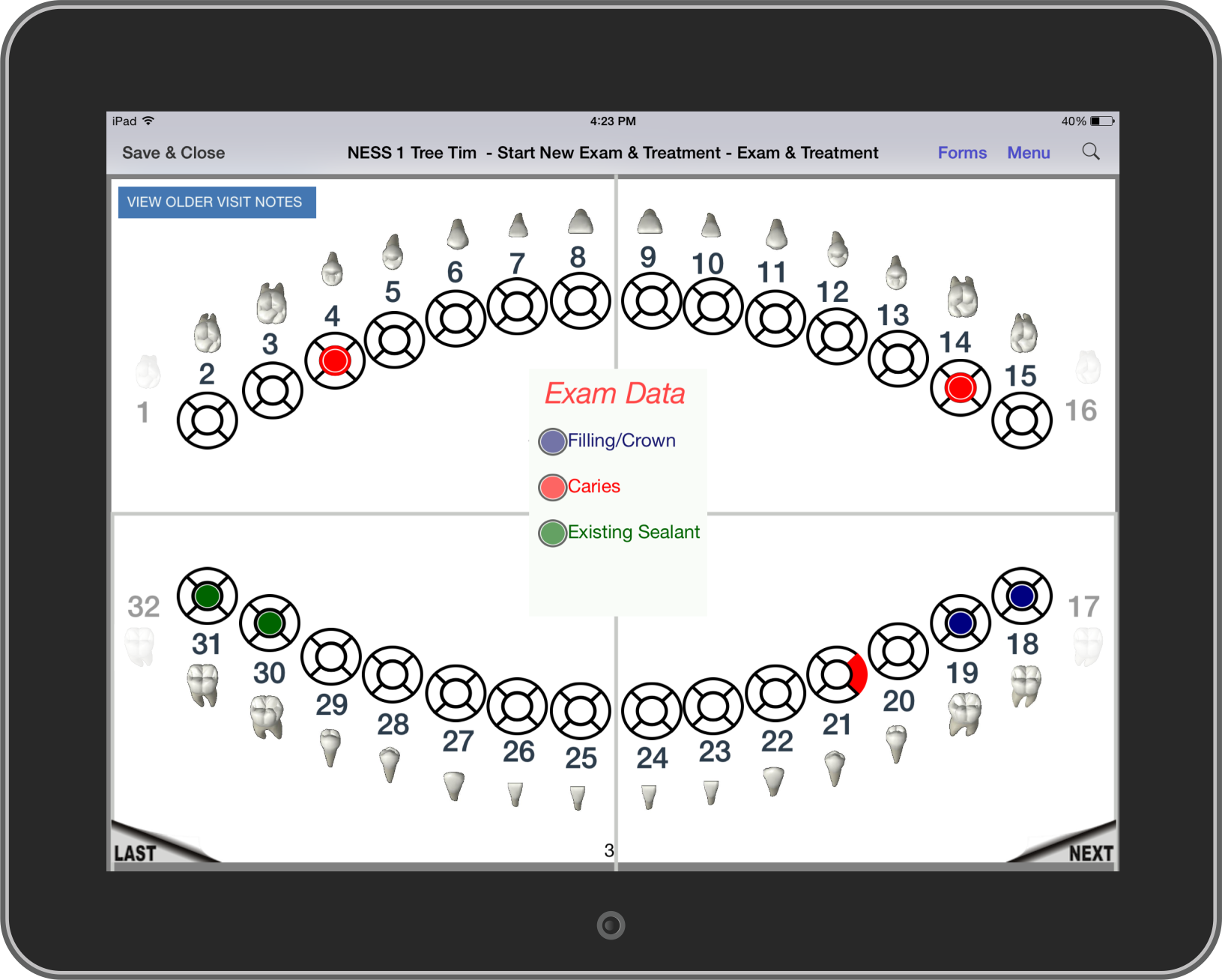
- Full dental charting with a user-friendly interface that dental professionals easily recognize
- Fast response time and intuitive controls allow dentists and hygenists to use their time more efficiently
- Forms are customized to the procedures being performed and exam data being collected
- Both the Universal Numbering System and Federation Dentaire Internationale Numbering System are available so dentists can use
their preferred notation
Back to top